Tristel ULT® High Level Disinfectant Foam For Ultrasound Probes
FDA APPROVED - 2 MINUTE CONTACT TIME - TRUSTED WORLDWIDE
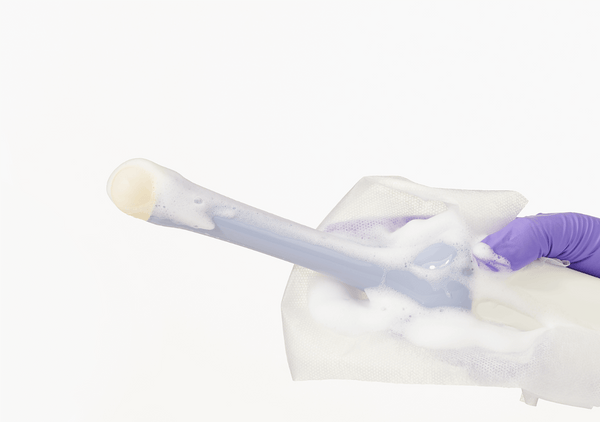
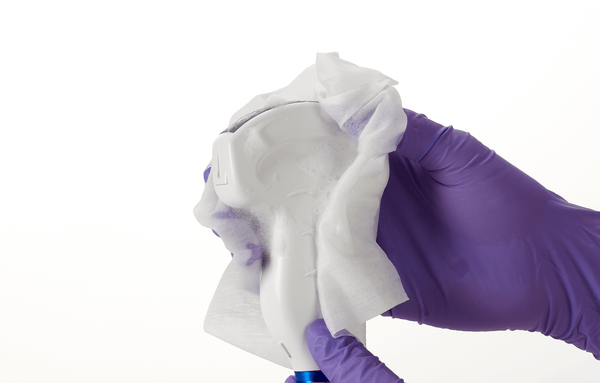
Tristel ULT is a high level disinfectant foam for reprocessing ultrasound probes. Tristel ULT can be used to high level disinfect endocavity transvaginal and transrectal probes, and skin surface transducers that may contact non-intact skin during use.
Used by professionals in 35 countries since 2008, Tristel ULT is now FDA-approved for sale in the United States. Tristel ULT is a prescription device under 21 CFR Part 801.109, classified as a Class II device under the generic name foam or gel chemical sterilant/high level disinfectant.
Features
- The fastest kill time for a high-level disinfectant - 2 minutes at room temperature
- Inactivates HPV type 16 and type 18 and HIV
- Safe, compact, portable, closest to point-of-care, and ready-to-use
- Can be used on endocavity and transvaginal ultrasound probes as well as general purpose transducers
- Each set delivers 75 HLD cycles
- Unique design/dispensing system guarantees accurate dosing
- Doesn't require dedicated facilities, capital investment, electrical/plumbing connections, validation, service contracts, or reserve units
- Deemed compatible with 965 ultrasound transducers.
- Click here for the Tristel Product Training Portal
Tristel™ ULT is manufactured and marketed in the United States by Parker Laboratories, Inc. as licensed by Tristel.
Specifications
| Mfr. No. 45-25 |
1 dual compartment bottle and 1 tub of wipes and a User Guide. Each set delivers 75 HLD cycles |
| Mfr. No. 45-13 |
Tristel Test Strips starter pack: 1 vial/bottle of 50 test strips, stopwatch, polyetheiene container, 10ml measure syringe, User Guide, 1 per case |
| Mfr. No. 45-10 |
Tristel Test Strips: 1 vial/bottle of 50 test strips, User Guide, 1 per case |