a-Chymotrypsin from bovine pancreas Type II, lyophilized powder, =40 units/mg protein
a-Chymotrypsin from bovine pancreas Type II, lyophilized powder, =40 units/mg protein
CAS Number 9004-07-3
EC Number 232-671-2
Enzyme Commission (EC) Number 3.4.21.1 ( BRENDA | IUBMB )
MDL number MFCD00130481
Analysis Note
Protein determined by E1%/280
Biochem/physiol Actions
A serine protease that hydrolyzes peptide bonds with aromatic or large hydrophobic side chains (Tyr, Trp, Phe, Met, Leu) on the carboxyl end of the bond.
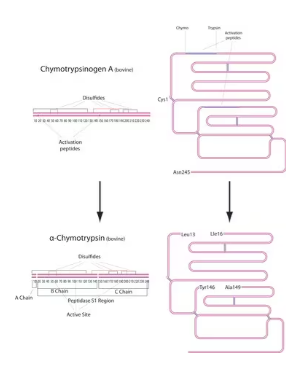
α-Chymotrypsin is a serine peptidase and has 241 amino acid residues contained in three polypeptide chains (A chain-13 residues, B chain-131 residues, and C chain-97 residues) linked by disulfide bridges. Molecular weight of this enzyme is found to be 25 kDa. The pI is 8.75. It selectively hydrolyzes peptide bonds on the C-terminal side of tyrosine, phenylalanine, tryptophan, and leucine. Ca2+ activates and stabilizes the enzyme. The enzyme is inhibited by diisopropyl fluorophosphate (DFP), phenylmethanesulfonyl fluoride (PMSF), N-p-tosyl-L-phenylalanine chloromethyl ketone (TPCK), chymostatin, aprotinin, α1-antitrypsin, and α2-macroglobulin, 10 mM Cu2+ and Hg2+.
Preparation Note
Derived from New Zealand-sourced pancreas
Produced from 3× crystallized chymotrypsinogen
Unit Definition
One unit will hydrolyze 1.0 μmole of BTEE per min at pH 7.8 at 25 °C.
Application
The enzyme from Sigma has been used to study the structure-function relationship in glycosylated α-chymotrypsin using immobilized metal-ion affinity chromatography (IMAC) and immobilized metal-ion affinity capillary electrophoresis (IMACE).
| Quality Level | PREMIUM |
| type | Type II |
| form | lyophilized powder |
| mol wt | mol wt 25 kDa |
| composition | protein, ≥85% |
| color | white to off-white |
| storage temp. | −20°C |
| Gene Information | cow ... CTRB1(618826) |